Electric charges are all around us. They are responsible for many of the phenomena we see, including the true nature of the normal force. Some charges can move and some can reorient to create interesting effects.
Lecture 1 | Micro-model of Charge, Q-transfer, Conductors vs. Insulators
Charges & the Electric Force
Lecture 1 | Micro-model of Charge, Q-transfer, Conductors vs. Insulators
Charges & the Electric Force
Pre-lecture Study Resources
Watch the pre-lecture videos and read through the OpenStax text before doing the pre-lecture homework or attending class.
Electric Phenomena | Micro-model of Charge and Q-transfer
All of the forces we experience on a daily basis, except gravity, are due to the electric force. The electric force is a fundamental feature of charged particles. Everyday matter is largely comprised of these particles - the positively charged proton and negatively charged electron. If you're sitting in a chair, the force preventing you from falling towards the center of the Earth is due to repulsive force of the electrons on your bottom with the electrons on your seat. The electrons can't part ways and slide past each other, which would cause you to fall to the floor, because of their attraction to the protons in the nucleus. This balance of repulsion and attraction, is what causes all of the contact forces we experience (normal, friction, tension, buoyancy, etc.).
To understand how this works, we should start with some fundamental facts.
- Particles are either positive (protons), negative (electrons), or neutral (neutron).
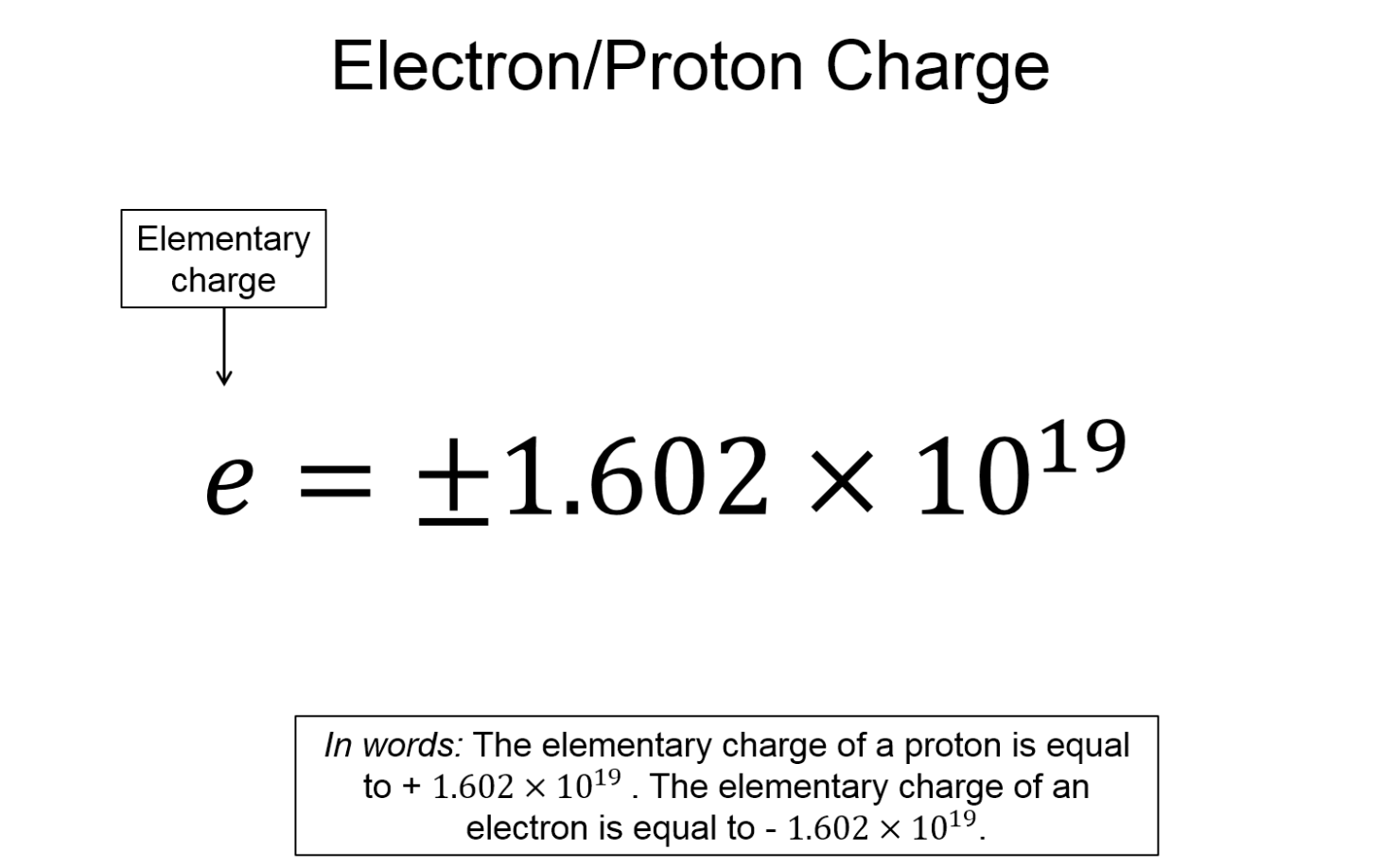
- Charges are quantized - the charge of a proton or electron is $e=\pm 1.602\times 10^{-19}$ coulombs.
- Total net charge of a system is, $Q_{net}=e(N_{protons}-N_{electrons})$, where N represents the number of protons or electrons.
- Like charges repel each other while unlike charges attract each other.
- Charges are transferable from one object to another.
- Insulators are material with no free electrons and thus charges cannot flow freely throughout the material. A Conductor is a material with free electrons (unbound) that can move freely, spreading out uniformly throughout the material's surface.
Conductors vs. Insulators
Most electrons are bound to a particular atom but there are some electrons that will be transferred to another atom or an entirely different object. Understanding how charge transfer works starts by understanding the two main types of materials, insulators and conductors. Both materials have fairly fixed positive ion cores (protons in the nucleus) that are much heavier (x1000) than the electrons surrounding them. Both have tightly bound electrons in the inner orbital shells.

Where they differ is in the presence of free electrons. Conductors have electrons that are bound enough to stay on the material but not bound too strongly to any one atom. They can move freely, allowing them to redistribute throughout an object quickly if initially concentrated in one location. Metals are often conductors and are used in electrical wiring. Insulators, in contrast, have no free electrons, and as such do not allow current to flow through them easily. The rubbery material surrounding the electrical wires are insulators to prevent you from getting shocked.
Charge Transfer
Electrons can be transferred between objects allowing for them to be in a net negative or net positive state. If two objects are initially net neutral, but after charge transfer occurs between them one is net positive, you know the other must be equally net negative. This is because one loses electrons and the other gains electrons. The protons (and neutrons) in the nucleus are not directly involved in any charge transfer, they are too far away and shielded from the action happening on the outskirts of the atom, where most of the important interactions occur.
Conductors can exchange charges by simply touching due to their free electrons. If touched, both objects will come to an equivalent equilibrium charge distribution. Consider two metal spheres, equivalent in every way except their charge, being brought into contact. If one was initially charged with a net charge $Q_{net}=+4 C$ and the other $Q_{net}=-2 C$, they have a net initial charge of $+2 C$. Since they have the same surface area, they will split the net charge equally, $+1 C$ each. The change will happen very rapidly (fractions of a fraction of a second), with the free electron charges transferring between objects and redistributing evenly. If the shape and size of the objects differ, they tend towards having the same surface charge density.
Insulators can have some charge transfer by simple contact but friction is often required for any large effects. Take a plastic rod and some cat fur and rub them together and one will lose electrons while the other gains them. Which one gains or loses an electron depends on the relative electronegativity of each material. Insulators differ from conductors in that the exchange of electrons only effects the region where the touching occurred. Since there are no free electrons to redistribute the excess charges, only the top half of the plastic rod would display charged effects if that was the only half rubbed.
Key Equations and Infographics
Required Videos