Thermo | 1st Law of Thermodyamics, Energy Transfers
If you recall the conservation of mechanical energy stated that work (W) is a mechanism for changing the energy of a system.
$E_i + W = E_f$, which can be rearranged to state, $W = \Delta E$
Although this is sufficient to study mechanics, it falls short when thermal energy is involved or when energy is transferred via heat - stand next to a fire and you can feel the difference in the energy when compared to far away. It is the heat (Q) transfer you're feeling that increases your internal thermal energy. The Kinetic Theory of Gases introduces thermal energy (Eth) as proportional to the temperature. So the first thing we need to do is expand our energy model to include thermal energy. (In fact there are other energies that we will add later, such as the energy to create/annihilate a particle, Einstein's's famous $E=mc^2$)
$E_{total}=K_{linear}+K_{rotational}+U^g + U^s + E_{th}+...$
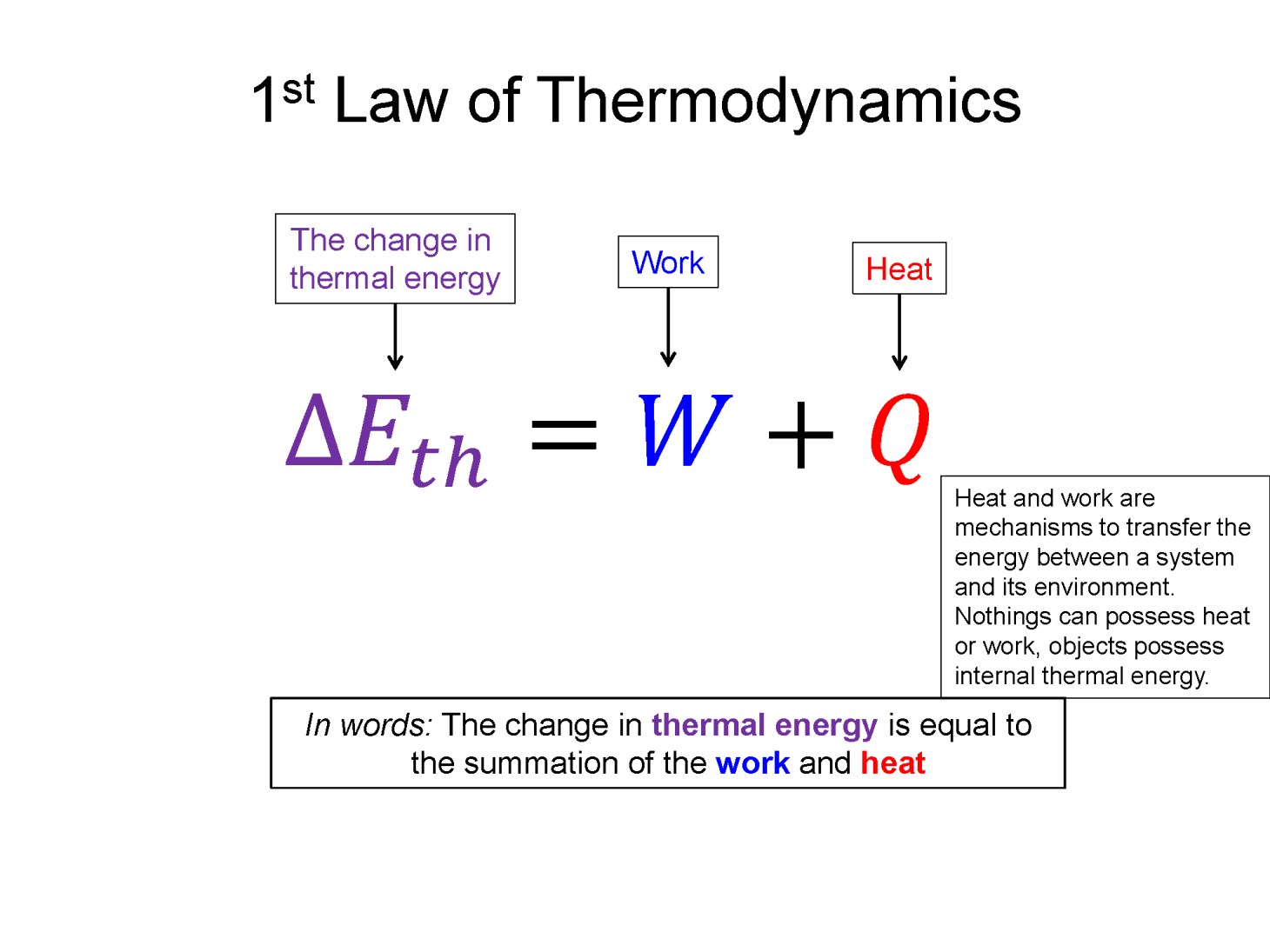
Now with this expanded view of energy we can imagine not only changing the energy of the system via work, but also through heat. The first law of thermodynamics is this energy transfer statement: $\Delta E_{total} = Q + W$. Now since most thermodynamic problems do not involve using heat to change the collective kinetic or potential energy of a system but rather the temperature (microscopic random kinetic energies of the individual particles), the only relevant energy is the thermal energy. So the most common form of the first law of thermodynamics is the following.
$\Delta E_{th} = Q + W$
What's incredibly important here is that nothing possess heat or work, they possess internal thermal energy. Heat and work are simply mechanisms to transfer the energy between a system and its environment. Colloquially people often say things like, "that fire's got a lot of heat", which in a physics view is an incorrect statement. The fire contains a lot of thermal energy, as shown by the high temperature, and it is transferring a lot of that energy to the environment via the mechanism of heat. If the environment the fire is in is at the same temperature, there would be zero heat transfer. An example of using work to change the thermal energy of a system would be rubbing your hands together and feeling them warm up.
Key Equations and Infographics