Fluid Statics | Pressure
Consider comparing the differences between gold and Styrofoam (polystyrene foam). Perhaps you wish to compare the weight of two materials. Well, you can obtain one pound of gold and one pound of Styrofoam, so perhaps weight is not a good quantity when trying to compare the two materials. But now that you have a pound of each material, you notice that pound of gold takes up much less volume than the pound of Styrofoam. Cleverly, you define a new quantity called mass density, which is the mass per unit volume of each material. Mathematically this is written as…
$\rho = \frac{m}{V}$
It turns out, that the density is a material property. One pound of gold has the same density 2 pounds, 1,000 pounds, 1/100th of a pound, etc..
We must be a little bit more careful though. Consider water, if we place a container of water under enough pressure, we can slightly change the density. Likewise, if we change the temperature of a container of water, the density also slightly changes. Thus, the density of any material is dependent on temperature and pressure. However, for solids and liquids, the density is very nearly constant for a wide range of temperatures and pressure, thus throughout our fluid mechanics studies, we will ignore these small density variations due to temperature and pressure changes. Another way to state this approximation is to say that we will assume fluids are incompressible (i.e. their densities are constant for any temperature or pressure).
While standing on a horizontal patch of ice, the normal force that you apply on the ice is equal to the force of gravity from the earth on you ($m \, g$). The ice supports this normal force no matter how you stand on the ice (e.g. lay down or stand with high heels on). However, you might already known that you would feel much more comfortable lying down on thin ice than standing with high heels on. Even though the ice is supporting the same weight ($m \, g$), the ice is more likely to break if you wear the high heels, thus we must introduce a new quantity to differentiate between these two scenarios. The new quantity introduced is called pressure. Pressure is defined as the perpendicular component of force divided by the area that the force is applied to. Mathematically this is written as…
$P = \frac{F_{\perp}}{A}$
Notice how it is only the perpendicular component of force. Since area is a scalar and the perpendicular component of force is a scalar, then pressure must also be a scalar.
The SI unit for pressure is $\frac{N}{m^2}$, which is given a special name called a Pascal (Pa).
$1 Pa = 1 \frac{N}{m^2}$
*NOTE: Pressure is often written as $P = \frac{F}{A}$, where it is implied that the force is only the perpendicular component.
Pressure plays an important role in fluid mechanics because pressure differences cause an associated net force.
$pressure \, difference \Longrightarrow \sum{\vec{F}}$
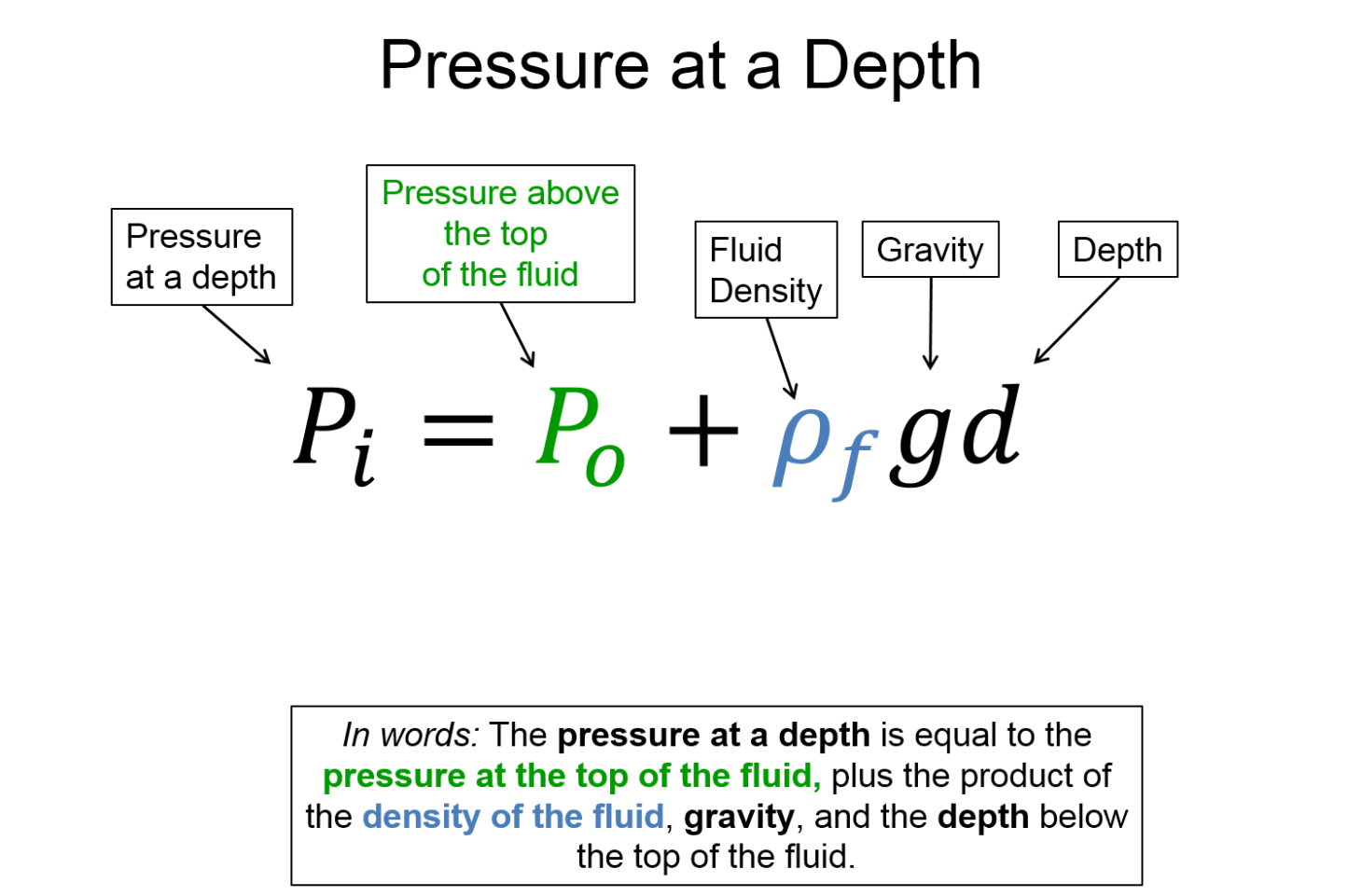
As we will see in the hydrostatics section, there exists a pressure gradient within a fluid near a massive object like the Earth - the pressure increases the deeper you go in the fluid. So pressure and the associated net force due to pressure differences are the foundations upon which we will build our fluid mechanics models.
Atmospheric pressure ($P_{atm}$)
We live in a mixture of gasses (the atmosphere) that surrounds us in all directions. Consider a column of atmosphere directly above us. This column of atmosphere has an associated weight, and just like our ice example, this atmospheric weight is supported by the surface of the Earth, or our heads as we stand on the Earth’s surface. Thus if we consider the force that this column of atmosphere applies over the area of the column, we can define a pressure due to the atmosphere near the Earth’s surface. The actual atmosphereic pressure is complicated, depending on temperature, height, humidity, etc… However, at sea level in standard conditions according to the International Standard Atmosphere model (ISA), the standard atmosphereic pressure is $101.325 \, kPa$. This value is also defined as 1 atmosphere ($atm$).
$1 \, atm = 101.325 \, kPa$
Gauge pressure ($P_{g}$)
The devices we use to measure pressure within a closed container often measure the pressure difference between the inside of a container and the outside. For example, if you use a tire pressure gauge to measure the pressure in your car tires, you are really measuring the difference between the outside atmosphereic pressure and the total pressure inside the tire (as seen in the figure below).
Absolute pressure ($P_{abs}$)
The absolute pressure is then the sum of the atmospheric pressure and gauge pressure.
$P_{abs} = P_{atm} + P_{g}$