Lecture 1 | Micro View of Matter, Kinetic Theory of Gases
Microscopic Model of Gases
Lecture 1 | Micro View of Matter, Kinetic Theory of Gases
Microscopic Model of Gases
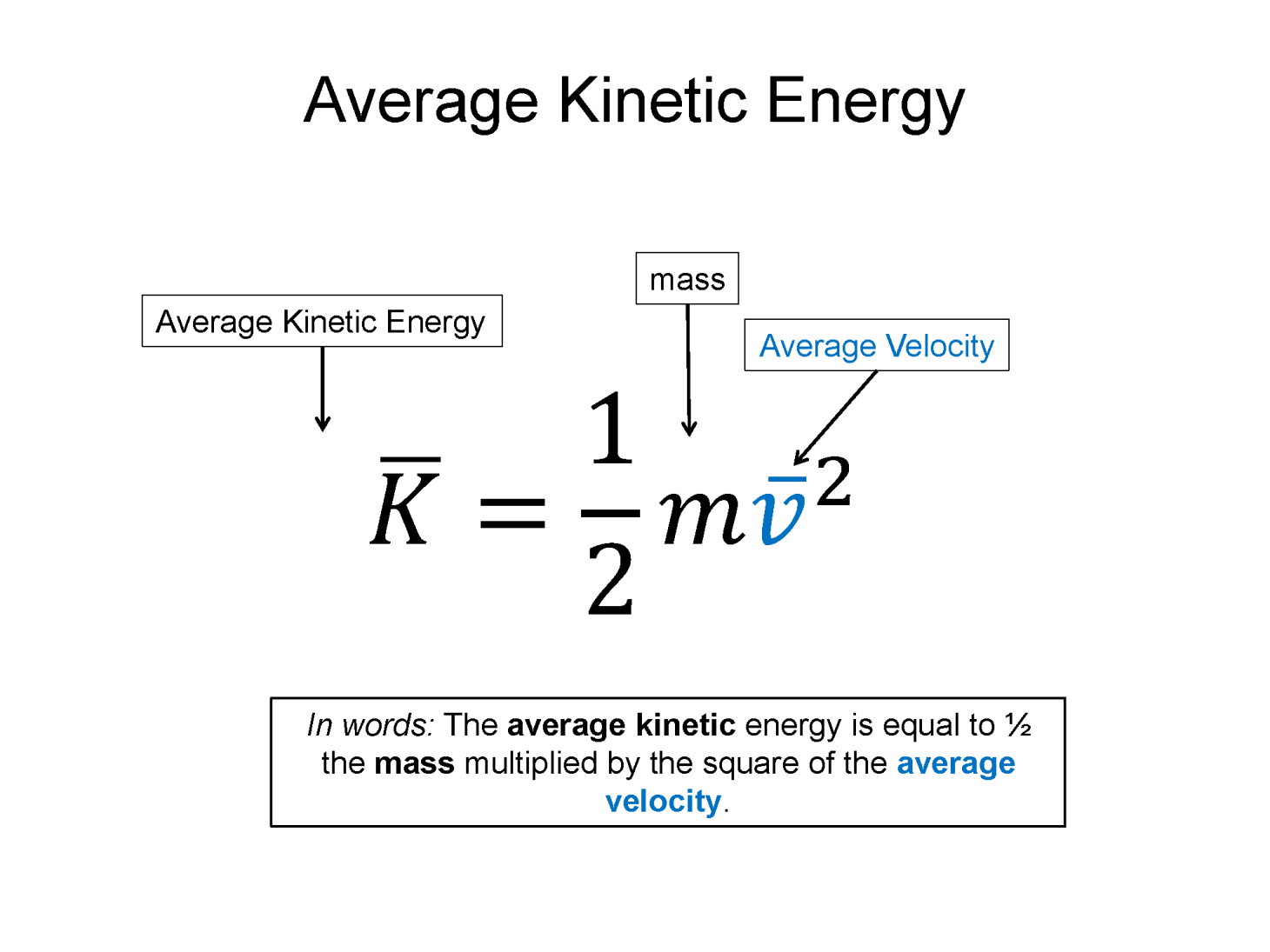
Matter comes in three main states, solids, liquids, and gases. The kinetic theory of gases models a gas as a collection of seldom interacting particles, flying around in random directions. The molecules of the gas have a range of speeds, due to their occasional interaction. For a complete energy analysis this motion of the particles of the gas needs to be accounted for. Thermal energy is what we call the summation of all the individual kinetic energies of the molecules of the gas. As the kinetic energy increases, the temperature increases. The kinetic theory connects the average speed of the particles with the temperature and kinetic energy.
Check out this quick introduction to kinetic theory from Doodle Science.
Pre-lecture Study Resources
Watch the pre-lecture videos and read through the OpenStax text before doing the pre-lecture homework or attending class.
Thermodynamics | Kinetic Theory of Gasses
The Kinetic Theory of Gases is an important model that paves the way for connecting a very microscopic view of matter to the very macroscopic measurements we make. The model treats a gas as a collection of microscopic particles traveling with a distribution (Maxwell-Boltzman) of speeds, all moving in random directions, bouncing off each other and the walls of the container holding the gas. The distribution of speeds is specific to a given macroscopic temperature.
OpenStax Section 13.1 | Temperature
OpenStax Section 13.4 | Kinetic Theory: Atomic and MOlecular Explanation of Pressure and Temperature